Introduction
Colorectal cancer (CRC) is a type of cancer that primarily affects the colon and rectum, which are parts of the large intestine. This form of cancer begins with the development of noncancerous growths called polyps on the inner lining of the colon or rectum. Over time, some of these polyps can transform into colorectal cancer. It's one of the most common types of cancer worldwide and a leading cause of cancer-related deaths [1].Colorectal cancer ranks as the third most prevalent cancer worldwide, contributing to approximately 10% of all cancer cases in 2020, there were approximately 1.93 million new cases and 0.94 million deaths due to colorectal cancer globally.The incidence of CRC is expected to rise significantly by 2040, with estimates suggesting a 63% increase in new cases and a 73% increase in deaths [2].
Colorectal cancer treatment integrates multiple modalities tailored to disease stage and specific patient factors. Surgery is typically the first-line treatment for localized colorectal cancer, meaning cases where the disease has not spread to other organs. Surgery aims to remove the cancerous tissues, and if the cancer is caught early, it can be highly effective. However, surgery is less effective in cases where the cancer has spread (metastasized) to other parts of the body [3]. For more advanced cases, Chemotherapy, including combinations like FOLFOX and FOLFIRI, plays a crucial role, particularly in advanced stages, although side effects and varying effectiveness pose challenges [4]. Targeted therapies such as EGFR inhibitors (e.g., cetuximab, panitumumab) are employed based on specific genetic markers in tumours, but mutations like KRAS can limit their efficacy [5]. Radiation therapy is another option, typically used alongside chemotherapy or for palliative care in advanced stages, restricted by its potential toxicity. Lastly, immunotherapy benefits patients with certain genetic markers like MSI-H or dMMR, offering significant advantages to those responsive to immune checkpoint inhibitors. Each treatment option balances benefits and limitations, necessitating personalized approaches based on detailed genetic and molecular tumour profiling [4]. Fruquintinib is a selective inhibitor of vascular endothelial growth factor receptors (VEGFR) 1, 2, and 3. It works by targeting and inhibiting the action of these receptors, which play a crucial role in the angiogenesis process that supplies blood to tumours. By blocking these pathways, fruquintinib hampers tumour growth and proliferation, making it an effective treatment option for conditions like metastatic colorectal cancer.Fruquintinib has been approved in China and recently by the FDA for use in specific mCRC cases [6].
The treatment of colorectal cancer imposes a significant economic burden both through direct medical costs and indirect costs such as lost productivity. Direct costs include hospital stays, chemotherapy, radiation, and surgical procedures. In the U.S., colorectal cancer has substantial financial implications, with significant out-of-pocket costs for patients, which can lead to financial toxicity. For example, patients often face high annual costs for treatments like chemotherapy, which can be as high as $5,600 per year [7]. Managing these costs within healthcare systems is challenging due to the expensive nature of cancer care and the need for prolonged treatment for many patients. Advanced therapies and the need for ongoing care, including primary treatment and follow-up, and management of side effects drive the high costs [8].This systematic literature review aims to provide a comprehensive assessment evaluating the cost-effectiveness of Fruquintinib for colorectal cancer. It helps determine whether these new treatments provide value relative to their costs, considering both their efficacy in extending quality-adjusted life years and their impact on healthcare expenditures.This analysis is vital for healthcare decision-makers, enabling informed decisions regarding the inclusion of new treatments in clinical practice, which can ultimately influence health policy and funding allocations.
Review Question
What is the cost-effectiveness of Fruquintinib for treating colorectal cancer compared to standard therapies, in terms of Incremental Cost-effectiveness Ratio (ICER), Quality-adjusted Life Years (QALYs), and overall healthcare costs?
Methods
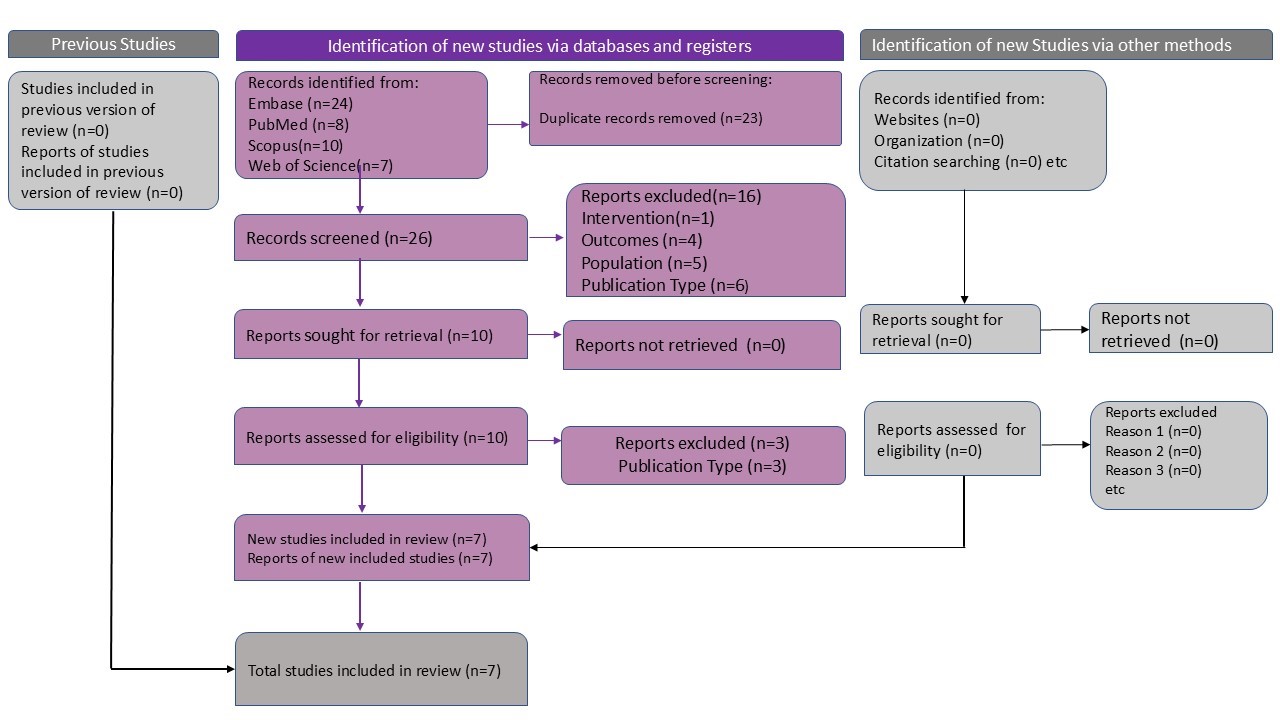
Search Strategy
This systematic literature review conforms to the highest standards of quality and transparency,