Introduction
Human Papillomavirus (HPV) infection is largely to blame for the rising incidence rates of Head and Neck Cancers (HNC), which represent a serious worldwide health concern. About 4.5% of all cancers worldwide are caused by HPV, which accounts for 630,000 new cases each year. Women are more likely than men to be infected with HPV (8.6% vs. 0.8%). It is most commonly linked to cervical cancer, which is responsible for 83% of cases linked to HPV. But, particularly in more developed nations, HPV also has a major effect on oropharyngeal and other anogenital malignancies. The study finds that most of these malignancies are associated with HPV types 16/18, along with types 6/11/16/18/31/33/45/52/58, based on data on the prevalence of HPV in cancer tissues [1]. Because of unique biological activities that affect patient outcomes, HPV-positive malignancies typically have a better prognosis than HPV-negative ones [2].
With increasing incidence rates associated with HPV infection, HNC represent a significant worldwide health concern. HPV causes about 4.5% of all malignancies worldwide, translating to 630,000 new cases per year. The frequency is higher in women (8.6%) than in men (0.8%). Although HPV is primarily responsible for 83% of cervical cancer cases, it also significantly affects other anogenital and oropharyngeal malignancies, especially in more industrialized nations. The majority of these malignancies are associated with HPV types 16/18 and a combination of HPV types 6/11/16/18/31/33/45/52/58, according to data on the frequency of HPV in cancer tissues used to evaluate attributable fractions and HPV type contributions. Our understanding of oropharyngeal cancers has evolved due to HPV's notable association with these diseases. Approximately 70% of oropharyngeal cancers in the US are caused by HPV, distinguishing them from other HNCs linked to alcohol and tobacco use [3]. HPV-positive malignancies usually have a better prognosis than HPV-negative ones due to differing biological activities affecting patient outcomes [2].
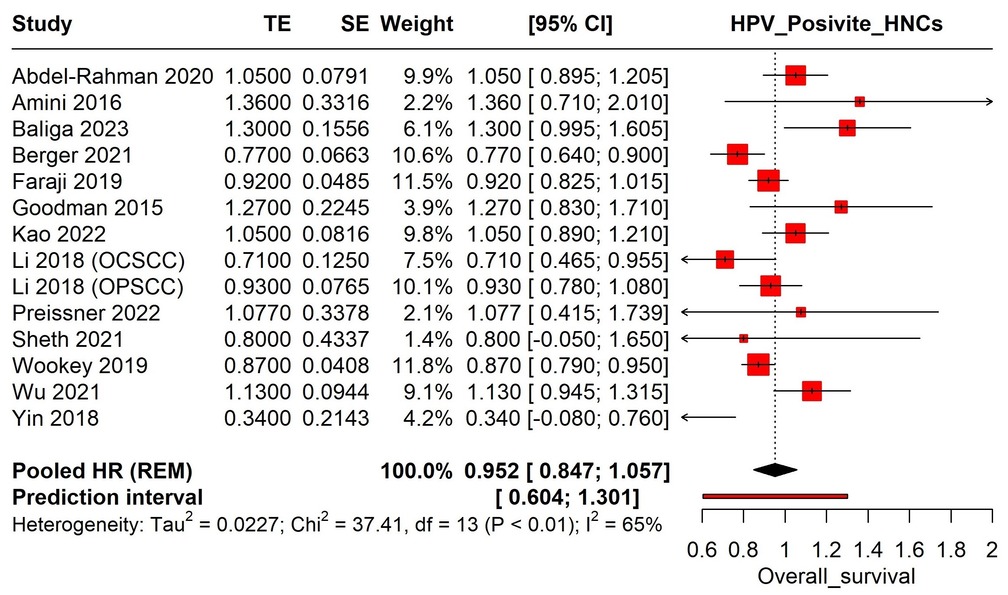
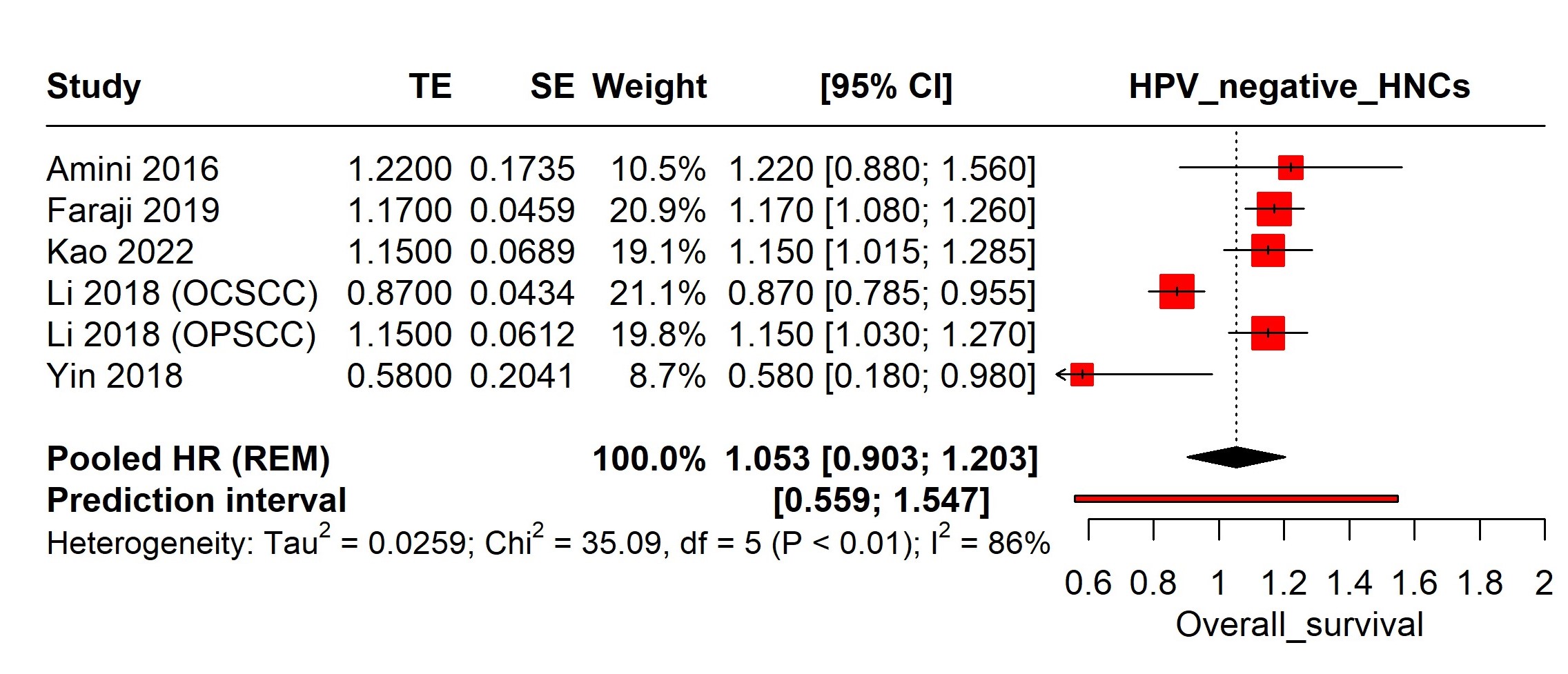
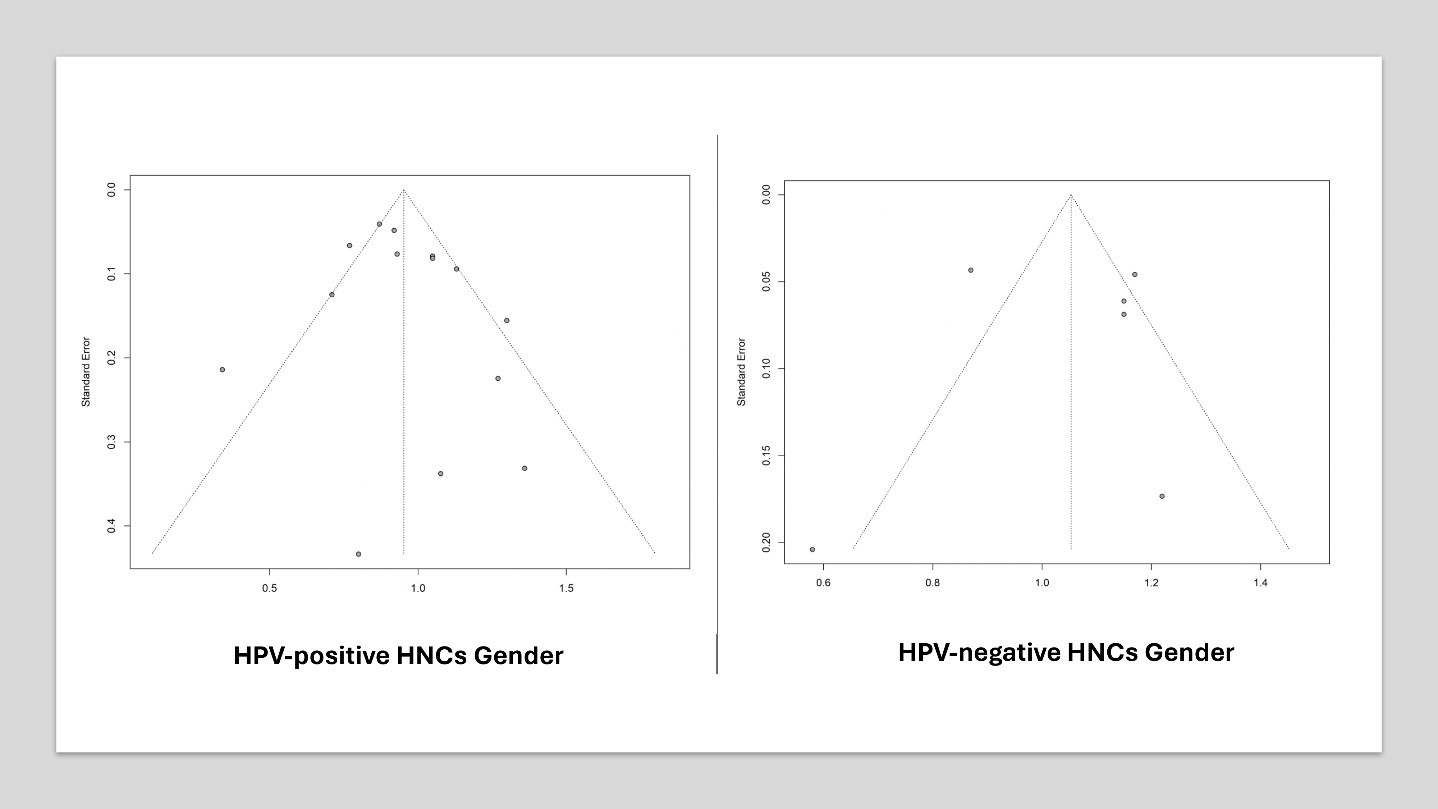
In high-income countries, the incidence of HPV-related oropharyngeal cancers is substantial, with variations across sex, race, and age [4]. Gender disparities in survival rates for HPV-positive HNC persist, with studies showing significant differences in overall survival between males and females. Recent research indicates that the incidence of HPV-associated Oropharyngeal Squamous Cell Carcinoma (OPSCC) is more than twice as high in men compared to women [5-10]. Understanding the impact of gender on survival rates in HPV-associated HNC is crucial for effective patient management and public health strategies to reduce cancer-related disparities. Examining these demographic factors is essential for developing tailored interventions and improving outcomes for all individuals, ensuring both men and women receive optimal, personalized care based on their specific needs and risk factors [11, 12].
This systematic review and meta-analysis evaluated the existing literature on the impact of gender on survival outcomes in HPV-associated HNCs to identify significant trends and underlying mechanisms that explain observed disparities. Focusing on these factors is crucial in the era of precision medicine, enhancing our understanding of diverse patient needs and potentially leading to more effective, personalized cancer treatment strategies.
Methods
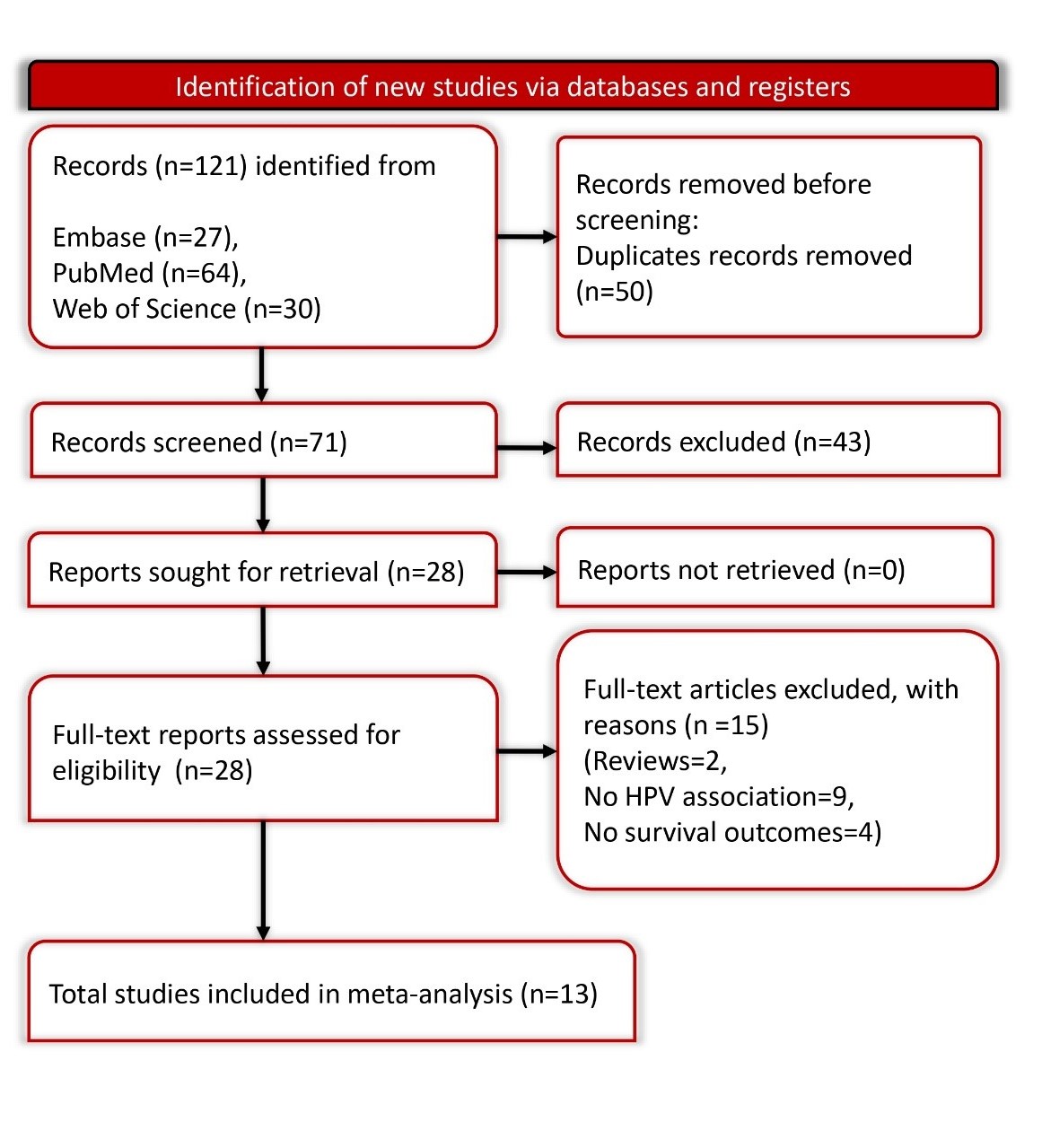
This review followed the PRISMA-2020 guidelines (Table S1) and the study protocol was registered with PROSPERO (CRD42024547847) [13].
Search Strategy
We executed an extensive search of the literature using multiple databases such as PubMed, Web of Science, and Embase, starting from their inception until May 2024. Our search methodology utilized a mix of keywords and Medical Subject Headings (MeSH) related to HPV, and head and neck cancer. Details of the search terms and strategies are outlined in Supplementary Table S2. There were no language or publication date restrictions, ensuring a comprehensive inclusion of pertinent studies.
Inclusion and Exclusion Criteria
Studies qualified for inclusion in our analysis if they: involved patients diagnosed with HPV-associated head and neck cancer, provided data on overall survival rates categorized by gender,