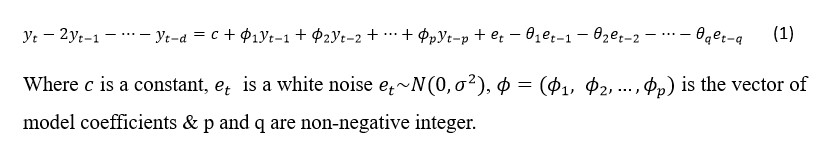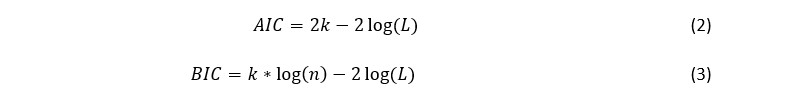affecting Central African regions with significant outbreaks reported in the Democratic Republic of Congo (DRC) [4, 5]. Clade II, encompassing subtypes IIa and IIb, is less virulent but more adept at spreading globally, as evidenced during the 2022 outbreak that significantly impacted non-endemic regions [6]. This distinction underscores the need for tailored public health strategies and global cooperation, particularly following the World Health Organization's 2024 declaration of Mpox as a Public Health Emergency of International Concern (PHEIC), highlighting persistent global health challenges [7]. The first declaration of Mpox as a PHEIC in 2022, and now again in 2024, underscores a persistent pattern of failures in the global response to epidemic-prone pathogens [8]. This second declaration by the WHO on August 14, 2024, highlights ongoing challenges such as vaccine disparities and significant gaps in understanding Mpox's epidemiology, transmission, pathogenesis, and control strategies [8].
The 2022 outbreak was characterized by atypical presentations, including genital lesions and sexual transmission, particularly among men who have sex with men (MSM), highlighting new transmission dynamics [3, 9]. The WHO reported 94,707 cases and 181 deaths globally by March 2024, with the United States accounting for a significant portion of these cases [9]. As of June 2024, the WHO has reported an outbreak with nearly 100,000 confirmed cases and 200 fatalities, primarily in the DRC [10]. Neighbouring countries such as Burundi and Kenya have also been impacted [10]. Furthermore, both Sweden and Thailand have each reported one case linked to travel. As of September 24, 2024, the WHO reported that there are more than 106,000 confirmed cases and 234 deaths in 123 countries [11, 12]. Despite the availability of vaccines and antiviral treatments like tecovirimat, challenges remain in their distribution and effectiveness, particularly in non-endemic regions [1, 3]. Public health measures such as surveillance, case isolation, and targeted vaccination are crucial, yet gaps in knowledge about the virus's animal reservoirs and mutations persist [3, 13]. The recent outbreaks underscore the need for a coordinated global response, integrating public health strategies, community engagement, and interdisciplinary research to prevent future outbreaks and mitigate the impact on vulnerable populations [13, 14]. The MpoxReC aims to enhance vaccine access and regulatory processes in Africa, advocating for the use of attenuated vaccinia-based vaccines during outbreaks [15]. The global response to Mpox must prioritize equitable access to medical countermeasures and address the socio-economic factors contributing to its spread [14, 15].
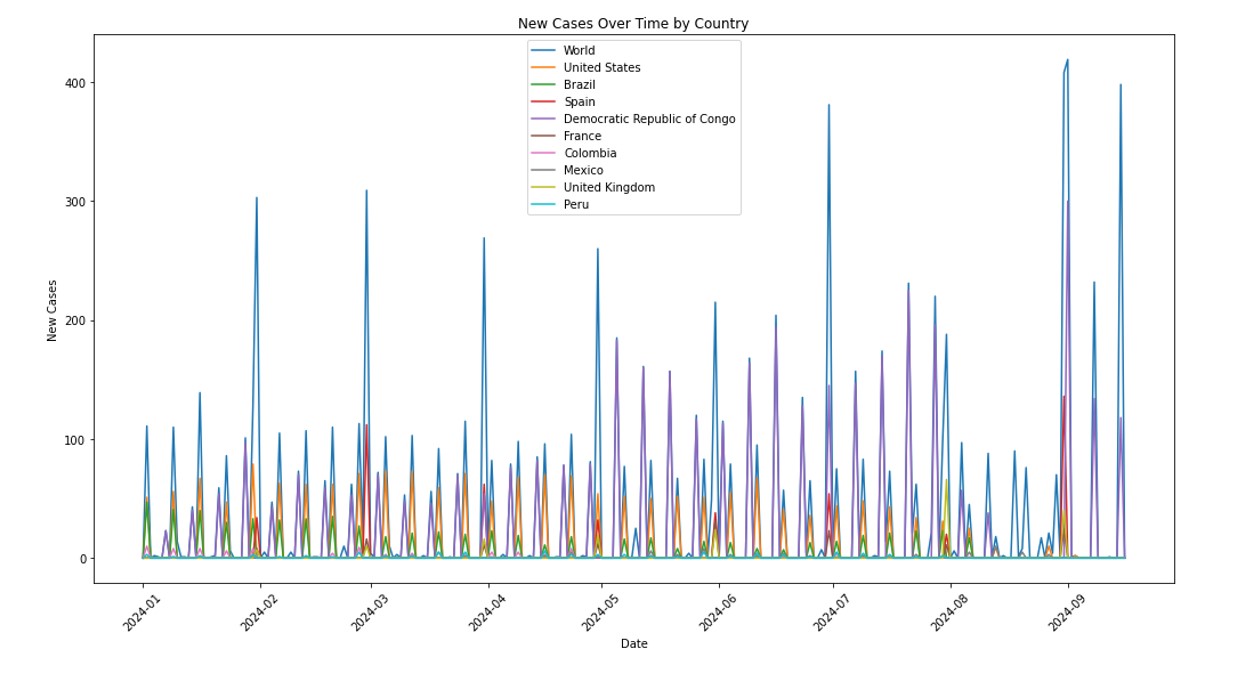
Mpox cases can be characterized as time-series data, displaying dynamic fluctuations under varying epidemic prevention and control scenarios. This variability underscores the suitability of time-series models for forecasting. The ability to predict daily new cases and total confirmed cases in the hardest-hit countries is critical from a practical perspective, as it informs effective public health decision-making and intervention strategies. Advanced methodologies like Deep learning architectures, such as Convolutional Neural Networks (CNN), Long Short-Term Memory (LSTM), and their combinations, have been employed to model the volatile and short-term data of Mpox cases, with the BaLSTM ensemble method achieving significant accuracy improvements over traditional models like ARIMA and SVM in Brazil [16]. The EpiNow2 model, used for nowcasting and forecasting in the U.S., demonstrated lower probabilistic error compared to Bayesian models, highlighting the importance of adapting models to different outbreak phases [17, 18]. Time series models like ARIMA and SARIMA have been applied to predict future Mpox cases, with SARIMA showing slightly better performance [18]. The modified SEIR model, adapted from COVID-19 predictions, has been used to simulate Mpox transmission and control scenarios, indicating the potential impact of vaccination on reducing case numbers [19]. Deterministic and stochastic models have been developed to understand Mpox dynamics, emphasizing the role of animal-to-human transmission and the basic reproduction number in managing outbreaks [20].
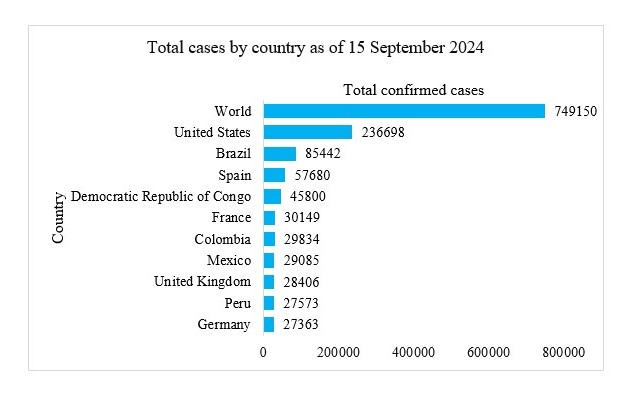
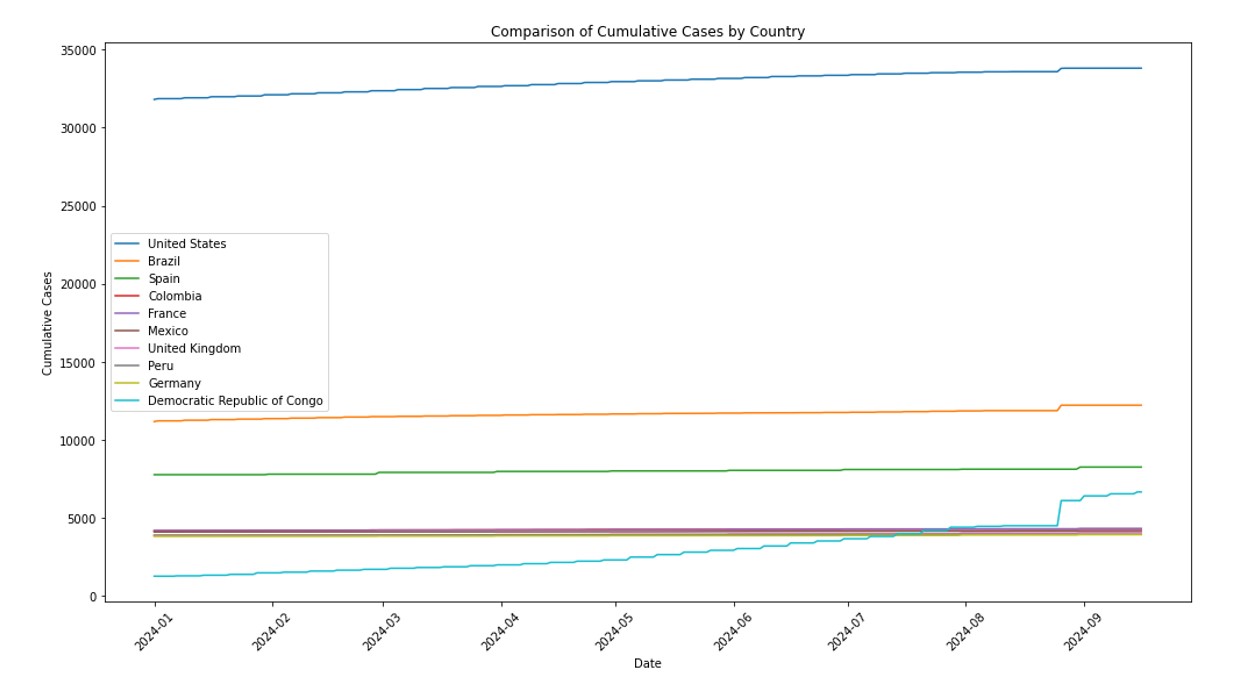
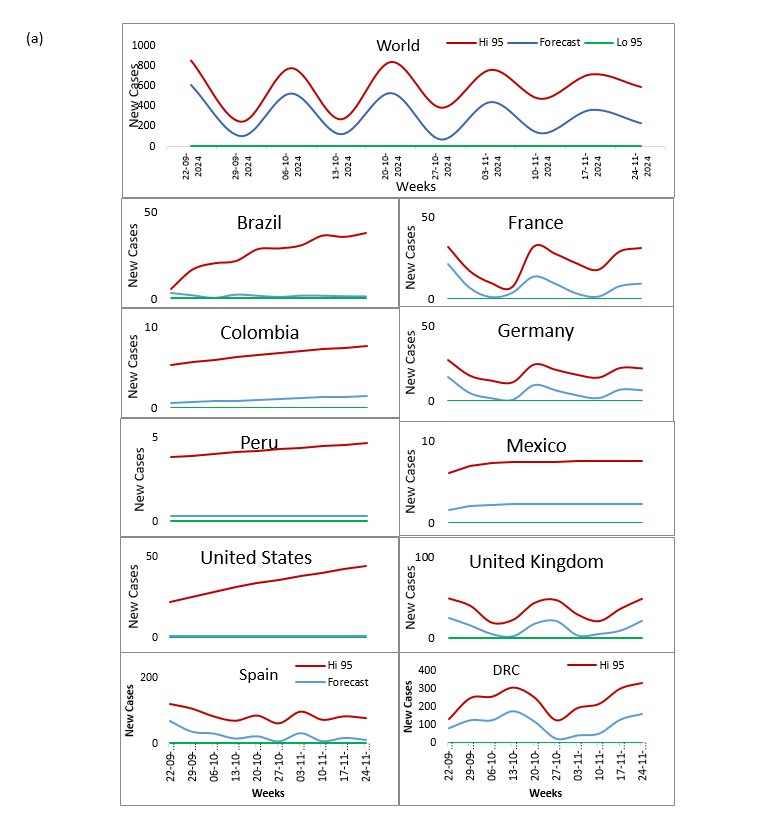
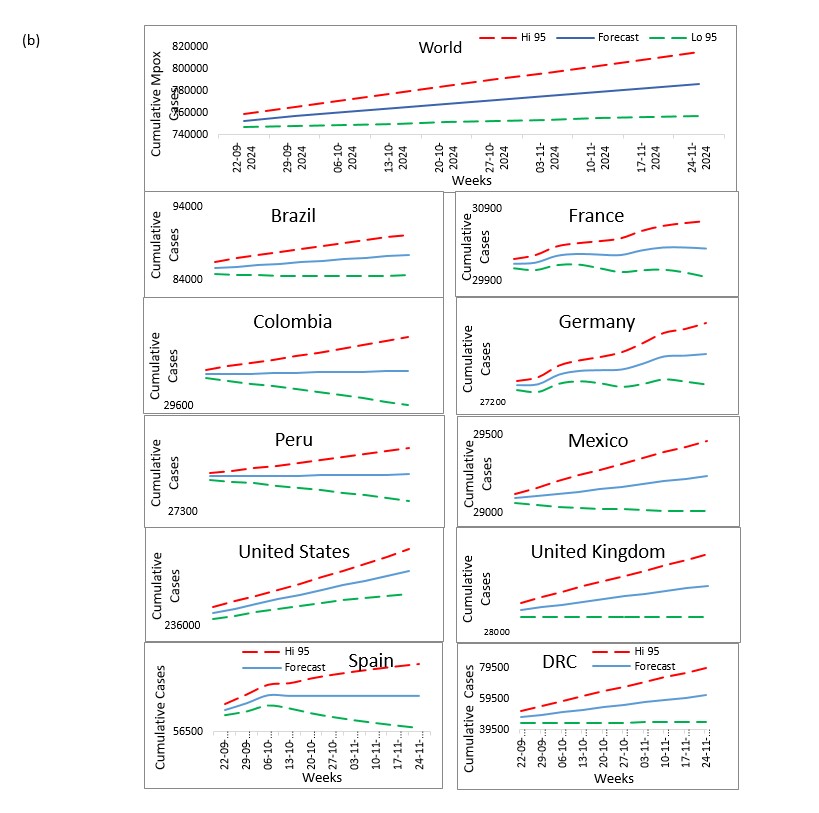
Our study aims to refine Mpox forecasting by integrating Jointpoint and ARIMA models, with a focus on accurately predicting disease trends and supporting effective public health responses. This approach combines the trend analysis capabilities of Jointpoint with the robust time-series forecasting of ARIMA to provide deeper insights into Mpox transmission dynamics. Utilizing data on daily confirmed and cumulative cases from the ten most affected countries United States, Brazil, Spain, Democratic Republic of Congo, France, Colombia, Mexico, United Kingdom, Peru, and Germany we aim to forecast the next 10 weeks.